Research ethics 2 column
The importance of ethics in research
Do you need ethical approval for your research?
The importance of research ethics.
Access Imperial's ethics code.
When in doubt
- Contact your Ethics and Research Governance Coordinator.
Seeking ethical approval
- Which ethical review body should you submit to?
- New process for non-NHS research ethics applications from July 2022: using an online Ethics Module via the Worktribe system which will enable users to submit their application via this platform.
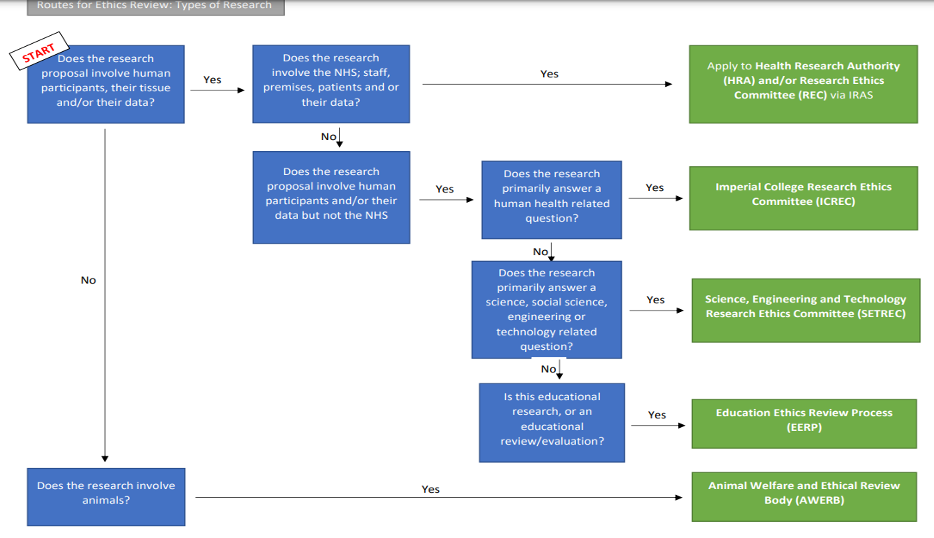
- Diagram: Routes to seeking ethical review at Imperial (see below)
Some Key Concepts in Application Forms
- Conflict of Interest: Policy that explains what it is and what to do when it may arise.
- Levels of Risk in Research: Some application forms (ICREC & SETREC) will ask you to determine the level of risk in your research
Low Risk: the research may cause minor discomfort or inconvenience, but it will not be greater than what they would be exposed to in their daily lives (e.g. the research involves collecting non-sensitive information).
Medium Risk: the researcher or participant are in an environment where there is potential risk of harm or discomfort (e.g. an institute with vulnerable people). In these cases, appropriate steps would need to be taken to mitigate harm and reduce the risk (e.g. no lone working).
High Risk: there is a likely risk of psychological or physical discomfort or harm if not managed in a responsible manner. Such research may involve intimate details of vulnerable participants, and/or highly sensitive topics.
Research ethics 2 column
Application forms
Application forms & guidance according to what your research involves
- Animals and no human participants, you need ethics review from Animal Welfare and Ethical Review Body (AWERB).
- NHS (staff, premises, patients and or their data), you need ethics review from the Health Research Authority (HRA) and/or Research Ethics Committee (REC) via IRAS.
- Human participants (answering a human health related question), you need ethics review from the College’s Research Ethics Committee (ICREC).
- Human participants (answering a science, social science, engineering or technology related question), you need ethics review from the Science, Engineering and Technology Research Ethics Committee (SETREC).
- Human participants (educational research, answering a pedagogical question), you need ethics review from Education Ethics Review Process (EERP)
Possible outcomes of your application
Approved as submitted
The research ethics committee/RGIT do not raise any ethical issues and no amendments are required.
Approved with conditions
Some minor specified conditions or changes to the application are required. No resubmission is required.
Request amendments
Major changes are required (e.g. modifying the method for data collection), which will require the committee’s/RGIT approval before progressing. Returned for complete resubmission
Returned for complete resubmission
If the application does not provide enough details about the research and how it will be ethically conducted, or any supplementary documents are of unacceptable standards, then it will need to be resubmitted.