The trend towards automated high content assays has stimulated the development of automated fluorescence microscopy systems including motorised sample stages, auto-focusing and almost hands-free image acquisition and analysis with several such instrument platforms becoming commercially available. Typically, commercial instruments predominantly undertake fluorescence intensity imaging in one or more spectral channels to map the relative distribution and co-localization of labelled biomolecules. There is, however, growing interest in fluorescence-based readouts of protein-protein interactions (PPI), e.g. using FRET-based readouts, but, to date, this is not yet well established for HCA and drug discovery.
Of the many approaches to map FRET, the most widely used in HCA is spectral ratiometric intensity imaging, although polarisation-resolved imaging and fluorescence lifetime imaging (FLIM) are being explored. Spectral ratiometric FRET measurements, which compare the donor and acceptor fluorescence intensities, have the advantage that they can be easier to implement in terms of instrumentation and require fewer detected photons, therefore providing faster imaging than lifetime measurements. One drawback of this approach is the requirement to calibrate the spectral response of the optical system, which can include the sample itself (inner filter effect) and spectral cross-talk, e.g. direct excitation of the acceptor. For robust spectral FRET data, this calibration is usually implemented though measurements of control samples, labelled with donor only and acceptor only, as well as the sample under investigation, e.g.1. The need for calibration of the whole systems presents challenges for comparison of spectral FRET measurements across different platforms.
 Polarisation resolved imaging represents an alternative intensity ratiometric technique to map FRET, which exploits the decrease in fluorescence anisotropy of the acceptor in the presence of FRET, e.g.2. This enjoys similar advantages as spectral ratiometric intensity imaging in terms of rapid data acquisition but is also sensitive to spectral cross-talk, requires calibration to take account of polarisation cross-talk, and does not directly provide the FRET efficiency. Thus it is a qualitative rather than quantitative readout3 of FRET in HCA, e.g.4.
Polarisation resolved imaging represents an alternative intensity ratiometric technique to map FRET, which exploits the decrease in fluorescence anisotropy of the acceptor in the presence of FRET, e.g.2. This enjoys similar advantages as spectral ratiometric intensity imaging in terms of rapid data acquisition but is also sensitive to spectral cross-talk, requires calibration to take account of polarisation cross-talk, and does not directly provide the FRET efficiency. Thus it is a qualitative rather than quantitative readout3 of FRET in HCA, e.g.4.
Because fluorescence lifetime readouts of FRET only require the donor fluorescence to be measured, FLIM assays are generally not compromised by spectral cross-talk and are insensitive to the excitation and detection efficiencies and the impact of scattering and sample absorption. Thus fluorescence lifetime-based FRET measurements can be directly compared across different FLIM instruments. Fitting fluorescence decay profiles to an appropriate multiexponential decay model can directly provide information on the fraction of the FRETing donor population, e.g. for dose response curves. The robust nature of FLIM readouts, including of FRET, means that they may potentially be translated along the drug discovery pipeline from in vitro assays to animal models5. In spite of these advantages, however, FLIM has not yet been widely applied to HCA. This is partly due to a lack of available FLIM instrumentation for automated multiwell plate readouts. In academic research laboratories, FLIM is often implemented using laser scanning microscopes with TCSPCand the sequential pixel acquisition of this approach typically results in data acquisition times of 10’s - 100’s of seconds per field of view for live cell FLIM FRET – depending on the labelling density. Such image acquisition times are impractical for HCA and if the excitation intensity is increased to permit much faster imaging, there are significant issues with photobleaching and photodamage.
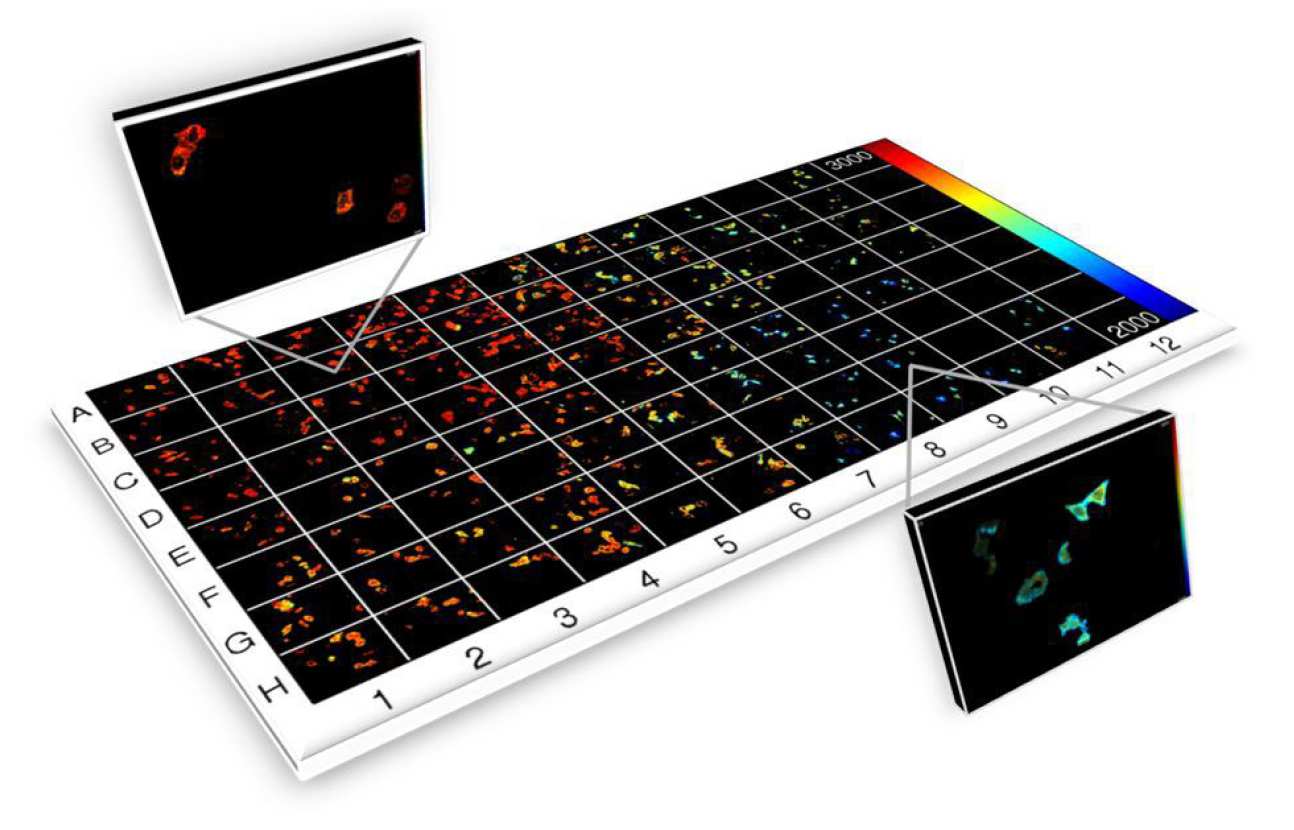
To realise rapid FLIM and FRET for HCA, it is necessary to parallelise the FLIM process and we have achieved this using wide-field gated image intensifiers to implement FLIM an automated multiwell plate reader that can also provide optical sectioning using a quasi-wide-field optically sectioning Nipkow (spinning disc) scanner6. This instrument has been developed as an open source HCA platform for automated (unsupervised) FLIM-FRET of fixed and live cells that can operate with acquisition times of less than 10 s per field of view, including sample translation, autofocus, cell finding and system calibration, and thus can read a 96 well plate in ~16 minutes (with one field of view per well), although we typically image multiple fields of view per well. It has been applied to a number of biological FRET-based assays, e.g.7, 8, and also to automated FLIM of cellular autofluorescence to assay changes in metabolic pathways , e.g. in response to anticancer drugs9.
1 Erickson, M. G., Alseikhan, B. a., Peterson, B. Z. and Yue, D. T., Neuron, 31 (2001) 973
2 Rizzo, M. A. and Piston, D. W., Biophysical Journal, 88 (2005) L14
3 Rizzo, M. a., Springer, G., Segawa, K., Zipfel, W. R. and Piston, D. W., Microscopy and Microanalysis, 12 (2006) 238
4 Matthews, D. R., Carlin, L. M., Ofo, E., Barber, P. R., Vojnovic, B., Irving, M., Ng, T. and Ameer-Beg, S. M., Journal of Microscopy, 237 (2010) 51
5 Kumar, S., Alibhai, D., Margineanu, A., Laine, R., Kennedy, G., McGinty, J., Warren, S., Kelly, D., Alexandrov, Y., Munro, I., Talbot, C., Stuckey, D. W., Kimberly, C., Viellerobe, B., Lacombe, F., Lam, E. W. F., Taylor, H., Dallman, M. J., Stamp, G., Murray, E. J., Stuhmeier, F., Sardini, A., Katan, M., Elson, D. S., Neil, M. A. A., Dunsby, C. and French, P. M. W., ChemPhysChem, 12 (2011) 609
6 Talbot, C. B., J. McGinty, D. M. Grant, E. J. McGhee, D. M. Owen, W. Zhang, T. D. Bunney, I. Munro, B. Isherwood, R. Eagle, A. Hargreaves, C. Dunsby, M. A. Neil, and P. M. French. J Biophotonics 1 (2008) 514
7 Alibhai, D., Kelly, D. J., Kumar, S., Warren, S., Serwa, R., Thinon, E., Alexandrov, Y., Munro, I., Margineanu, A., McGinty, J., Talbot., C. B., Murray, E. J., Stuhmeier, F., Tate, E. W., Neil, M. A. A., Dunsby, C. and French, P. M.W., J. Biophotonics 6, 398–408 (2013)
8 D. J. Kelly, S. C. Warren, D. Alibhai, S. Kumar, Y. Alexandrov, I. Munro, A. Margineanu, J. McCormack, N. J. Welsh, R. A. Serwa, E. Thinon, M. Kongsema, J. McGinty, C. Talbot, E. J. Murray, F. Stuhmeier, M. A.A. Neil, E. W. Tate, V. M. M. Braga, E. W.-F. Lam, C. Dunsby and P. M.W. French, Anal. Methods, 7 (2015), 4071-4089, DOI: 10.1039/C5AY00244C
9 D. J. Kelly, S. C. Warren, S. Kumar, J. L. Lagarto, B T. Dyer, A. Margineanu1, E. W.-F. Lam, C. Dunsby and P. M. W. French, “An automated multiwell plate reading FLIM microscope for live cell autofluorescence lifetime assays”, J. Innov. Opt. Health Sci. 7 (2014) 1450025-15 pages, DOI: 10.1142/S1793545814500254