Overview
ICTU is a UKCRC registered Clinical Trials Unit recognised for its experience and expertise in the development and delivery of clinical trials and associated high quality clinical research projects across a range of therapeutic areas. The unit leads primarily on the design, conduct and analysis of national and international multi-centre trials and the staff, with specialist disease and methodological knowledge, have input at all stages of the trial lifecycle; thus ensuring all activities are conducted according to the principles of ICH GCP and in compliance with the appropriate regulatory and ethical requirements.
ICTU sits within Imperial College London as a department within the School of Public Health in the Faculty of Medicine and brings together academic, clinical and trial management expertise from across the Imperial College Academic Health Science Centre. ICTU also collaborates with members of the designated Academic Health Science Network for North West London - Imperial College Healthcare Partners, other national key clinical and scientific opinion leaders, other CTUs and with industry.
Our expertise
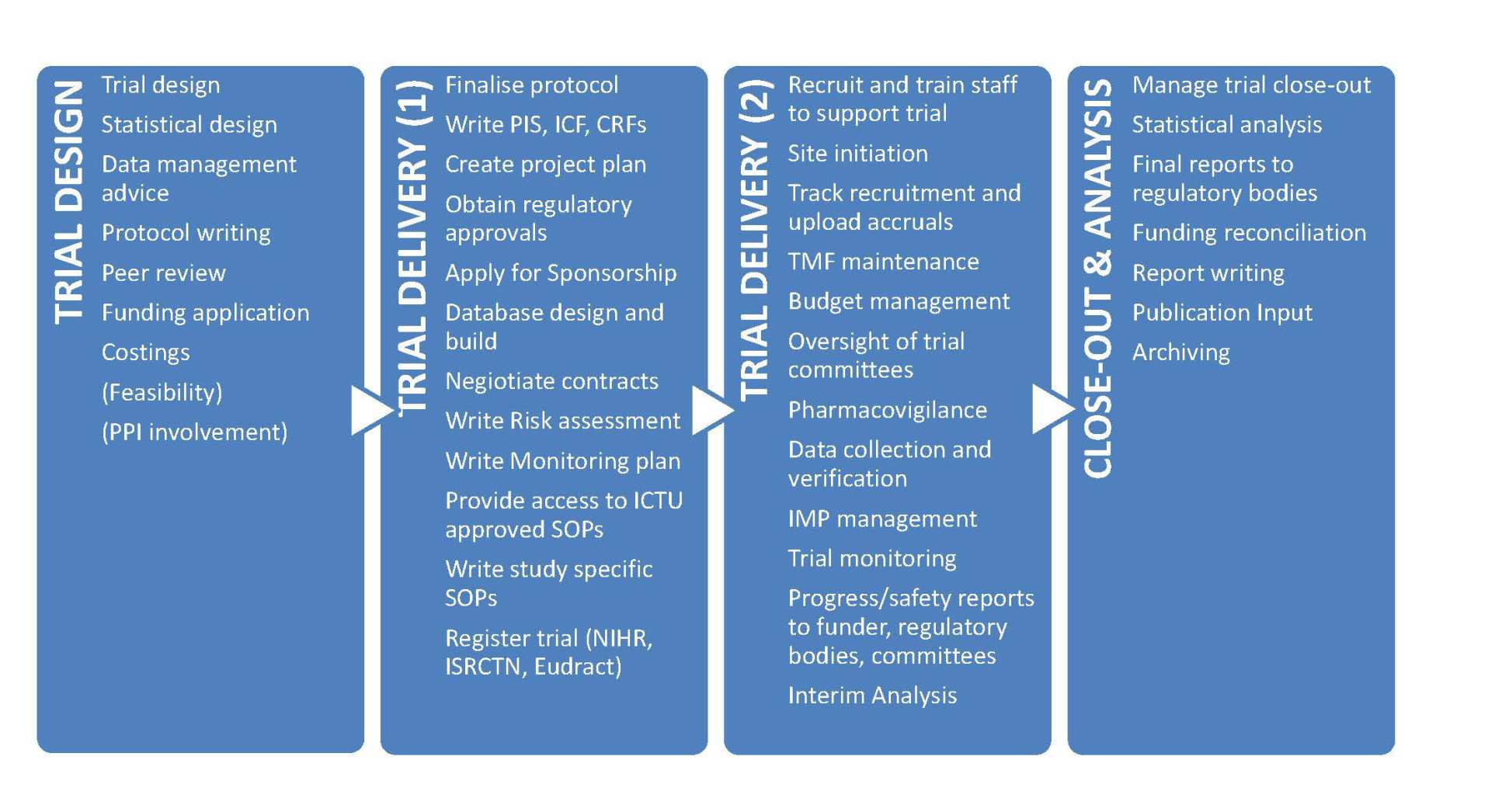
Each trial in the ICTU portfolio is allocated an ICTU study team according to the size and type of study, and whether full or partial collaboration has been agreed. On the operational side, a study team generally consists of a dedicated Trial Manager, Monitor and Clinical Trial Administrator, all overseen by an Operations Manager. A dedicated Study Statistician, with oversight from a Senior Statistician will be responsible for the statistical design and analysis of the trial. This team is then supported with central database development, data management and administrative support from the Clinical Data Systems team.
The team are responsible for all key trial coordination tasks such as:
 Additionally, ICTU has an in-house Quality Assurance team with responsibility for overseeing, maintaining and improving ICTU's Quality Management System.
Additionally, ICTU has an in-house Quality Assurance team with responsibility for overseeing, maintaining and improving ICTU's Quality Management System.
ICTU has a transparent and timely process for the review and prioritisation of proposals from investigators seeking collaboration with the unit. Proposals may entail complete or partial collaboration with ICTU. Visit our Collaborations page for more detailed information on how to collaborate with ICTU.
The Collaborations Section is currently being updated.
If your request falls under one of the Therapeutic areas details for the relevant email can be found on the Therapeutic Area Contact Details Page.
For commercial sponsors
Visit our dedicated website for commercial sponsors interested in working with ICTU.
