What is the TREAT trial?
The full name of the TREAT trial is: Treating severe paediatric asthma; a randomised controlled trial of mepolizumab and omalizumab. The study includes 2 parts: an observational (monitoring) phase and a treatment phase.
Why is the TREAT trial important?
Approximately 2-5% of children with asthma have persistent symptoms, which results in repeated asthma attacks despite being prescribed maximal treatments. Whilst it is recognised that these children should be assessed at specialist clinics there is currently no nationally agreed approach to their evaluation. It is also recognised that a large proportion have poor symptom control because they are not taking their prescribed treatments regularly. This can be addressed for some patients through the use of monitoring using electronic “smart” inhalers and education. However, there will still be, despite everyone’s best efforts, a group of children for whom asthma symptoms are troublesome and those children will be invited to take part in the second part of this study when they receive a medication to treat their asthma. These children have problematic severe asthma, the tubes (airways) in their lungs swell and get smaller due to the mucus in them.
In the second phase of the TREAT trial, we compare the two biologic treatments that are given by injection (Mepolizumab and Omalizumab) and are licenced for use in children, to see if both are equally good, or whether one works better for children with severe asthma. During the trial, we also collect lots of information which we hope will help us in the future, when deciding which treatment to give to an individual patient. The trial is taking place in 11 different hospitals across the UK, which means that together, we can come up with a national approach of how best to treat children with severe asthma.
The study design
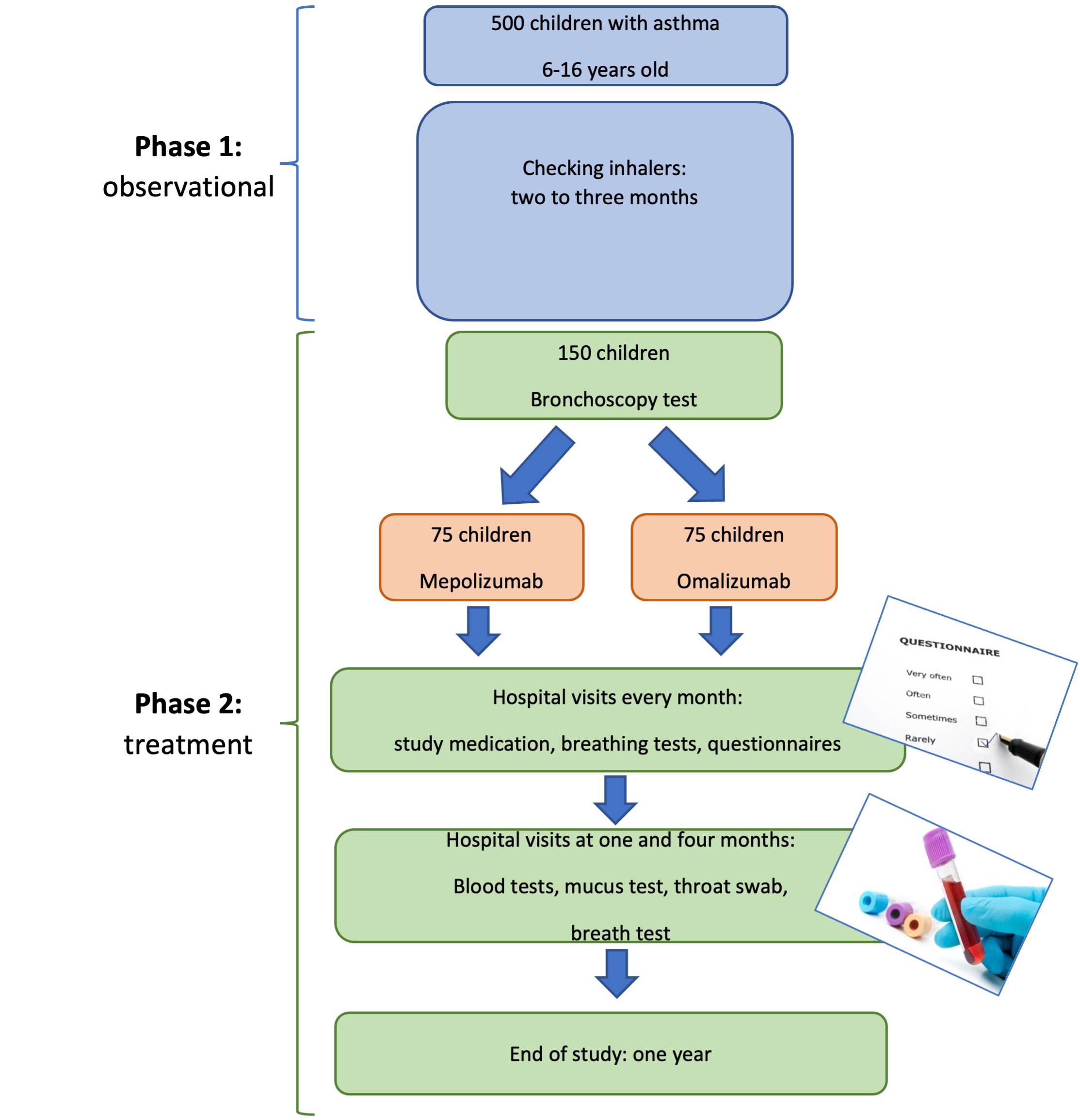
What is the purpose of the TREAT trial?
In this study, children with problematic severe asthma firstly receive a check to see if they are taking their inhaler treatment as prescribed, using electronic monitoring. All children that continue to have asthma attacks and persistent symptoms despite taking their inhalers, are eligible for the second phase whereby we compare the effectiveness of each treatment by assessing the number of asthma attacks the children have in that year. We are aiming to reduce number of attacks by one-third compared to the previous year. We also assess change in symptoms and the child’s and family’s quality of life. We want to know by the end of the study whether mepolizumab is at least as good as omalizumab in reducing asthma attacks in children with severe asthma.
What is the main endpoint of the trial?
To determine whether mepolizumab is as good as omalizumab in reducing asthma attacks in children with severe asthma.
Where is the TREAT trial taking place?
The TREAT trial is currently enrolling across 11 sites in the UK:
- Royal Brompton & Harefield NHS FT
- University Hospitals of Leicester NHS Trust
- King’s College Hospital NHS FT
- Birmingham Women's and Children's NHS FT
- NHS Lothian - Edinburgh
- NHS Greater Glasgow and Clyde
- University Hospital Southampton NHS FT
- Manchester University NHS FT
- Alder Hey Children's NHS FT - Liverpool
- University Hospitals of North Midlands NHS Trust - Stoke
- Brighton and Sussex University Hospitals NHS Trust
- Newcastle Upon Tyne Hospitals NHS Foundation Trust