Overview
Our researchers are working in collaboration with Rigel Pharmaceuticals and Novartis Pharmaceuticals on a clinical trial to evaluate the use of the drugs fostamatinib and ruxolitinib in patients with COVID-19 pneumonia. Launched in 2020 at Imperial College NHS Trust, the open-label trial is assessing whether hospitalized patients with COVID-19 respond better to the addition of fostamatinib or ruxolitinib to their standard of care. More specifically, the study will help researchers and clinicians to understand whether the drugs reduce the proportion of patients going on to develop severe pneumonia or die.
COVID-19 pneumonia can lead to respiratory and multi-organ failure caused by inflammation. Fostamatinib and ruxolitinib work by blocking different parts of the immune response. Both treatments are already in use for other diseases.
Below, you'll find further details about the trial, including specific information for clinicians and patients. You can also follow us on Twitter @MATISTrial for the latest updates.
Accordion widget
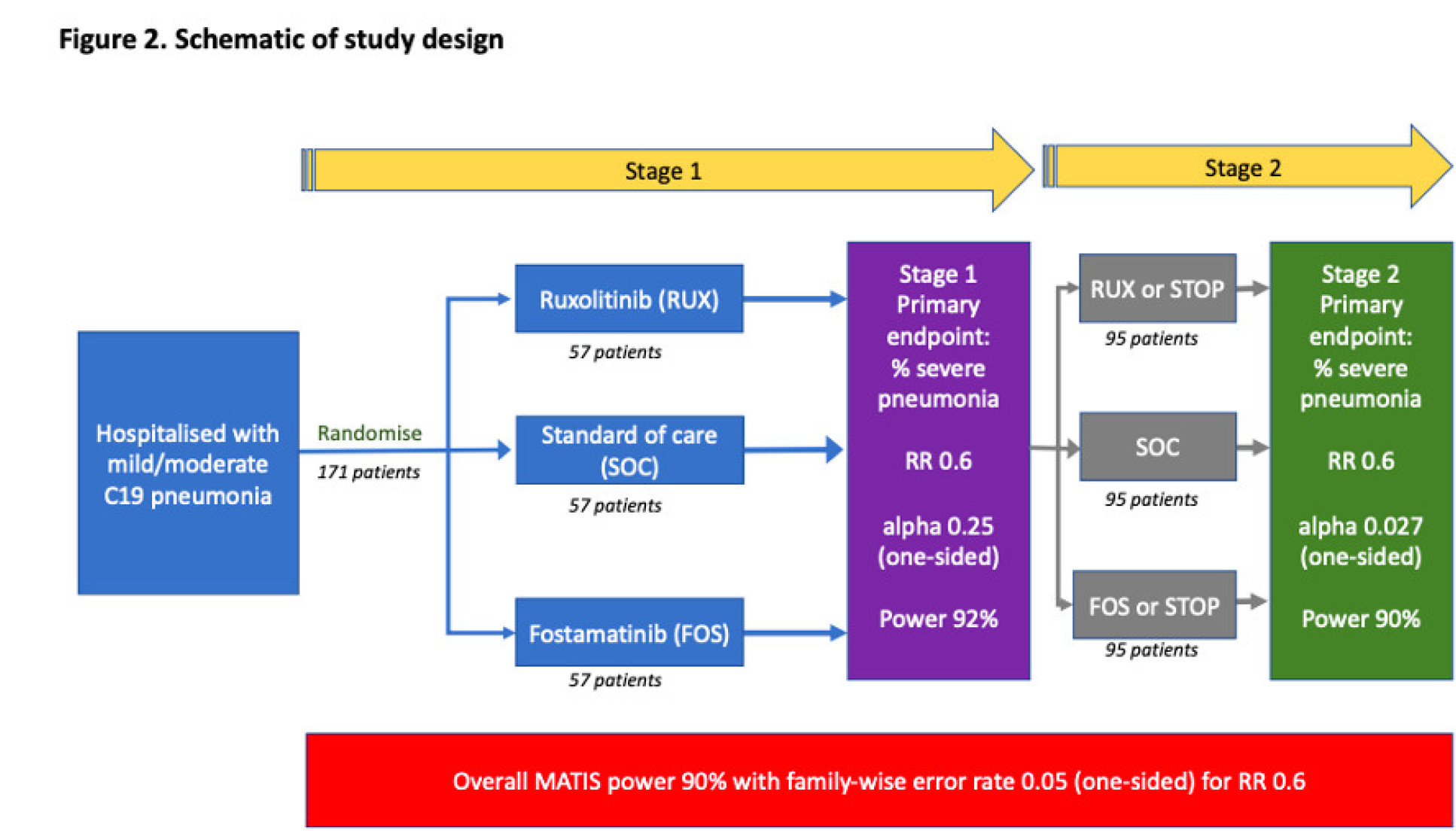
Study design
This is a multi-site, two-stage, open-label, randomized (1:1:1) controlled trial. Treatment is for 14 days from baseline.
Patients will be randomised to one of three outcomes:
- Receive fostamatinib in addition to standard of care
- Receive ruxolitinib in addition to standard of care
- Standard of care only
Patients will receive follow-up assessment at 7, 14 and 28 days after the first dose.
Patients who have recovered and are fit for discharge from hospital during the treatment period will be discharged home with trial medication to complete a fixed 14-day course.
Such patients will be followed up weekly with telephone monitoring until day 28 and additional blood test monitoring where practically possible.
Sample size
Stage 1: 171 (57 per arm) patients with COVID-19 pneumonia will be recruited to Stage 1
Stage 2: If the trial progresses to Stage 2 an additional maximum of 285 (95 per arm) will be recruited, resulting in a potential sample size of 456 if three trial arms continue for the whole trial (152 per arm).
Drug mechanism of action
FOSTAMATINIB (FOS)
- Fostamatinib is a tyrosine kinase inhibitor with activity against spleen tyrosine kinase (syk)
- It is licensed for use in refractory immune thrombocytopenia
- This evidence suggests SYK activity mediates cytokine and chemokine release, induced by the activation of C-type lectin receptors (CLR) and immunoglobulin Fc receptors (FcR), resulting in neutrophil and monocyte lung ingress. Leading to activation of neutrophil extracellular traps and activation of lung epithelium and multiple myeloid cells.
- This is followed by inflammation and tissue destruction hat contribute to ARDS
- By inhibiting SYK activity FOS can block the production and release of cytokines induced by CLR and FcE activation, thus potentially ameliorating the cytokine storm that often precedes ARDS
Fostamatinib is the only SYK inhibitor approved for clinical use, offering a unique anti-inflammatory therapeutic with a known safety profile
RUXOLITINIB (RUX)
- Ruxolitinib is a JAK1/JAK2 inhibitor approved for clinical use in the treatment of splenomegaly, myelofibrosis, polycythaemia vera and graft-versus-host disease.
- JAK and STAT molecules are proteins that transduce extracellular stimulation into intracellular signaling
- This leads to expression of a number of host inflammatory cytokines in a variety of immune cells
- It is an oral agent with a rapid mode of action
- Inhibition of STAT3 activation occurs within 2 hours of RUX administration
- Downregulates IL-6 and IL-23 signaling important for the proinflammatory effects of Th17 cells
- Further, RUX administration leads to in reductions in serum levels TNFa and CRP.
- JAK2 inhibitors have been shown to block receptor-mediated endocytosis, thereby preventing viral cellular entry and assembly
Information for clinicians
Using our understanding of the mechanisms of COVID-19 and knowledge of immuno-modulatory medications, we hope to find optimal treatments for COVID-19 infections.
- Ruxolitinib and Fostamatinib are well-known medications, which inhibit key signalling pathways which lead to the hyperinflammation associated with severe COVID-19.
- There may be an early window of opportunity to treat the COVID-19 hyperinflammatory syndrome before acute lung injury leads to organ failure.
- This is a randomised controlled, multi-arm trial of early intervention with inflammatory signal inhibitors.
- Elli, E.M., Baratè, C., Mendicino, F., Palandri, F., et al. (2019) Mechanisms Underlying the Anti-inflammatory and Immunosuppressive Activity of Ruxolitinib. Frontiers in Oncology. [Online] 9, 1186. Available from: doi:10.3389/fonc.2019.01186.
- Huang, C., Wang, Y., Li, X., Ren, L., et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). [Online] 395 (10223), 497–506. Available from: doi:10.1016/S0140- 6736(20)30183-5.
- Jagasia, M., Perales, M.-A., Schroeder, M.A., Ali, H., et al. (2020) Ruxolitinib for the treatment of steroidrefractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. [Online] 135 (20), 1739–1749. Available from: doi:10.1182/blood.2020004823.
- Richardson, P., Griffin, I., Tucker, C., Smith, D., et al. (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England). [Online] 395 (10223), e30–e31. Available from: doi:10.1016/S0140-6736(20)30304-4.
- Ruan, Q., Yang, K., Wang, W., Jiang, L., et al. (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine. [Online] 46 (5), 846–848. Available from: doi:10.1007/s00134-020-05991-x.
- Siddiqi, H.K. & Mehra, M.R. (2020) COVID-19 illness in native and immunosuppressed states: A clinical– therapeutic staging proposal. The Journal of Heart and Lung Transplantation. [Online] 39 (5), 405–407. Available from: doi:10.1016/j.healun.2020.03.012.
- Singanayagam, A., Glanville, N., Girkin, J.L., Ching, Y.M., et al. (2018) Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nature Communications. [Online] 9 (1), 2229. Available from: doi:10.1038/s41467-018-04574-1.
- MATIS version 1.9 dated 29 October 2020 IRAS: 282552 Page 49 of 50 Wu, D. & Yang, X.O. (2020) TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. Journal of Microbiology, Immunology, and Infection. [Online] 53 (3), 368–370. Available from: doi:10.1016/j.jmii.2020.03.005.
- Kost-Alimova et al. (2020) A High-Content Screen for Mucin-1-Reducing Compounds Identifies Fostamatinib as a Candidate for Rapid Repurposing for Acute Lung Injury. Cell Reports Medicine. Available from: doi:10.1016/j.xcrm.2020.100137.
- I.O. Rosas et al. (2020) Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. The New England Journal of Medicine. Available from: doi:10.1056/NEJMoa2028700.
Trial team members
Dr Nichola Cooper
/prod01/channel_3/media/migration/faculty-of-medicine/nikki-photo-copy-tojpeg-1515670701273-x1_1613052857994_x4.jpg)
Dr Nichola Cooper
Chief Investigator
Dr Richard Turner
/prod01/channel_3/media/migration/faculty-of-medicine/richard-turner_1613486772974_x4.jpg)
Dr Richard Turner
Principal Investigator
Dr Nikhil Vergis
/prod01/channel_3/media/migration/faculty-of-medicine/vergis-nikhil-19-03-2019-2-tojpeg-1580210171558-x1_1613486673878_x4.jpg)
Dr Nikhil Vergis
Principal Investigator
Dr Pratap Neelakantan
/prod01/channel_3/media/migration/faculty-of-medicine/28309-dun-1154647-mr-pratap-neelakantan-fa_1613493838340_x4.jpg)
Dr Pratap Neelakantan
Principle Investigator
Dr Victoria Parris
/prod01/channel_3/media/migration/faculty-of-medicine/blank-profile-picture-973460-1280-2_1613493698603_x4.jpg)
Dr Victoria Parris
Principle Investigator
Dr Ashley Whittington
/prod01/channel_3/media/migration/faculty-of-medicine/blank-profile-picture-973460-1280-2_1613493494640_x4.jpg)
Dr Ashley Whittington
Principle Investigator
Dr Anna Daunt
/prod01/channel_3/media/migration/faculty-of-medicine/blank-profile-picture-973460-1280-2_1613054129528_x4.jpg)
Dr Anna Daunt
Co-Investigator
Dr Andrew Innes
/prod01/channel_3/media/migration/faculty-of-medicine/unadjustednonraw-thumb-9af-tojpeg-1481755742085-x1_1613487030274_x4.jpg)
Dr Andrew Innes
Co-Investigator
Dr Dragana Milojkovic
/prod01/channel_3/media/migration/faculty-of-medicine/dragana-milojkovic-websize-1-1-cb3a33270aff0189ee73712478d37c38_1613487263060_x4.jpg)
Dr Dragana Milojkovic
Co-Investigator
Dr Lucy Cook
/prod01/channel_3/media/migration/faculty-of-medicine/blank-profile-picture-973460-1280-2_1613487403273_x4.jpg)
Dr Lucy Cook
Co-Investigator
Professor Graham Cooke
/prod01/channel_3/media/migration/faculty-of-medicine/img-1680-tojpeg-1570950554138-x1_1613492661148_x4.jpg)
Professor Graham Cooke
Co-Investigator
Dr Catherine Hockings
/prod01/channel_3/media/migration/faculty-of-medicine/blank-profile-picture-973460-1280-2_1613492986786_x4.jpg)
Dr Catherine Hockings
Visiting Researcher
Professor Onn Min Kon
/prod01/channel_3/media/migration/faculty-of-medicine/portrait_1613492767997_x4.jpg)
Professor Onn Min Kon
Co-Investigator
Professor Mark Thursz
/prod01/channel_3/media/migration/faculty-of-medicine/portrait_1613492574346_x4.jpg)
Professor Mark Thursz
Co-Investigator
Dr Taryn Youngstein
/prod01/channel_3/media/migration/faculty-of-medicine/ty-profile-1595502729704-x1_1613492471288_x4.jpg)
Dr Taryn Youngstein
Co-Investigator
Dr Victoria Cornelius
/prod01/channel_3/media/migration/faculty-of-medicine/cornelius-victoria-01-06-2018-2-copy-tojpeg-1535973313013-x1_1613568748896_x4.jpg)
Dr Victoria Cornelius
Statistician
Dr Rachel Phillips
/prod01/channel_3/media/migration/faculty-of-medicine/img-20180601-151417-2-1-1613559048446-x1_1613568891791_x4.jpg)
Dr Rachel Phillips
Statistician
Professor James Wason
/prod01/channel_3/media/migration/faculty-of-medicine/picturephp_1613493384896_x4.jpg)
Professor James Wason
Statistician

