The interaction of light with molecules continues to be a key area of research for the group. For example, photoswitchable molecules are photochemically active compounds that can be reversibly switched between (at least) two states by irradiation with light. There is high interest in developing new and improved photoswitches due to the spatial and temporal control made possible by using light as an external stimulus. As such, photoswitchable molecules are reported in numerous applications, from photopharmacology — where light switches between two states of a molecule with different pharmacological activities — to optical data storage.
Photochemistry
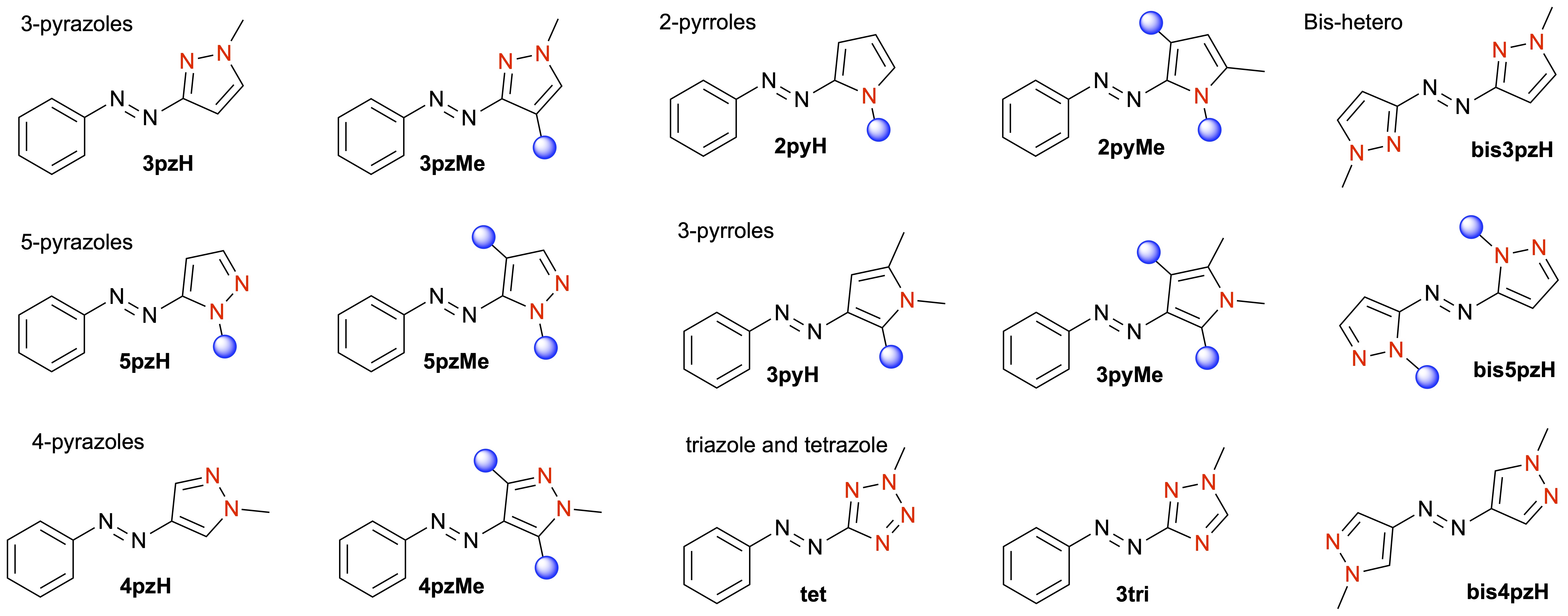
Azobenzenes represent a privileged scaffold for photoswitches, since they are easily synthesised, have high extinction coefficients and quantum yields (allowing photoswitching with low-intensity light), and are stable to repeated switching. However, photoswitching of azobenzene is often incomplete, with mixtures of E/Z isomers obtained at a given wavelength of light. We have been studying the replacement of one or both benzene rings in azobenzene with a heteroaromatic ring — an underdeveloped concept prior to our work. Our research has led to many exciting discoveries, particularly through the identification of switches that significantly outcompete azobenzene derivatives.
The most well-known switches we have developed are the arylazopyrazoles, which offer quantitative photoswitching in both directions and high thermal stability of the Z isomer (half-lives of up to 46 years). Beyond these molecules, we continue to explore the structure–property relationships for a wide array of comparable azoheteroaryl photoswitches, identifying compounds with Z isomer half-lives ranging from subseconds to years, and variable absorption characteristics — all through tuning of the heteroaromatic ring. Most recently, we have been using high-throughput computing and machine learning techniques to accelerate photoswitch discovery, allowing for tailored switch design for multiple optically addressable applications. Representative publications: Chem. Sci. 2022, 13, 13541. DOI; Beilstein J. Org. Chem. 2019, 15, 2753. DOI; J. Am. Chem. Soc. 2017, 139, 1261. DOI; J. Am. Chem. Soc. 2014, 136, 11878. DOI.
Leveraging our discovery of novel heteroaromatic azo photoswitches, we continue to explore their related applications. Beyond our developing work on photopharmacology, we have published on the use of our molecules as photoswitchable bases, in photoswitchable surfactants and within molecular cages. Representative publications: ChemRxiv 2023. DOI; JACS Au 2022, 2, 2670. DOI; Chem. Commun. 2016, 52, 4521. DOI.
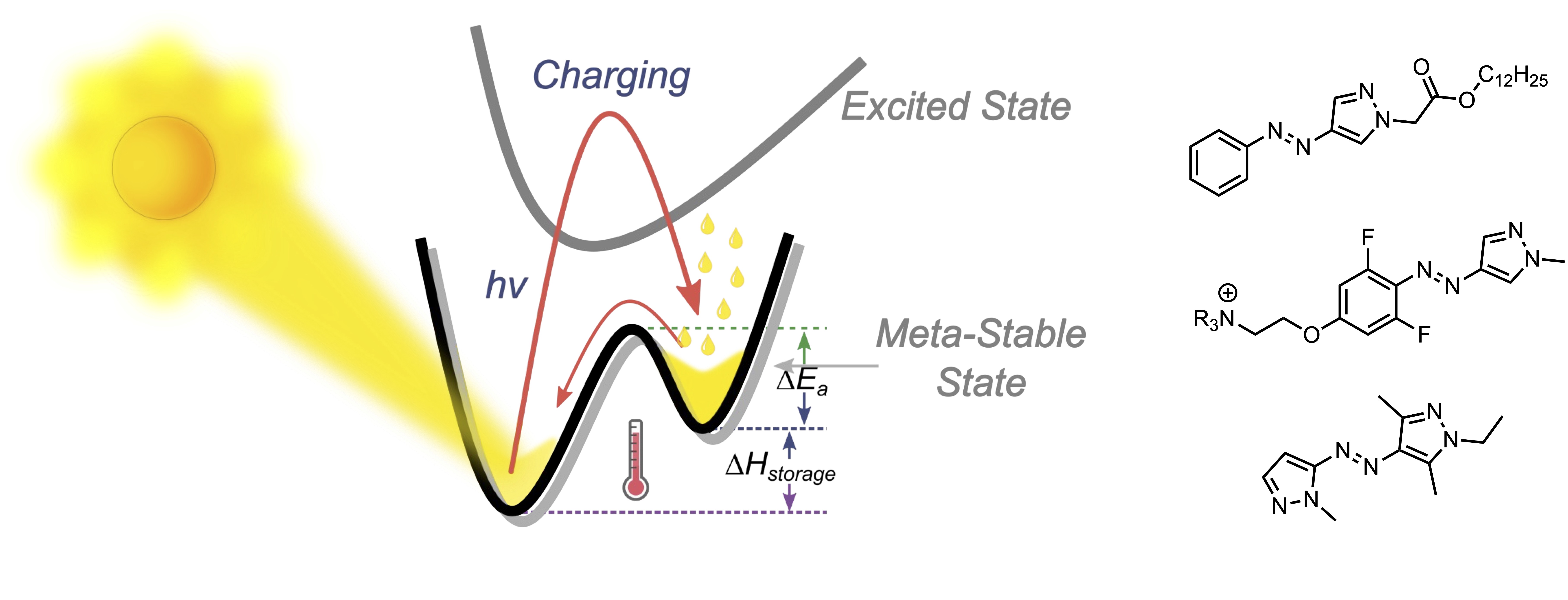 One area of recent interest has been in molecular solar thermal (MOST) energy storage materials. These photoswitchable materials convert photon energy to thermal energy by reversible isomerisation and energy storage in a metastable isomeric state. Such materials have high potential in applications ranging from waste heat recycling in vehicles and ‘off-grid’ homes, to heated clothing and other cold-climate amenities. We have been studying the arylazopyrazoles and azobispyrazoles in this regard, with several exciting outcomes. These include: (1) the discovery of a material that can store up to 92 kJ/mol of thermal energy for extended timescales (>2 weeks) while allowing for energy release at subzero temperatures, (2) the discovery of a material where energy release can occur electrocatalytically in the condensed phase, and (3) the discovery of materials with unprecedentedly large effective light penetration depths (1400 μm of UV at 365 nm and 1400 μm of visible light at 530 nm). Representative publications: J. Am. Chem. Soc. 2022, 144, 19430. DOI; J. Am. Chem. Soc. 2021, 143, 15250. DOI; J. Am. Chem. Soc. 2020, 142, 8688. DOI.
One area of recent interest has been in molecular solar thermal (MOST) energy storage materials. These photoswitchable materials convert photon energy to thermal energy by reversible isomerisation and energy storage in a metastable isomeric state. Such materials have high potential in applications ranging from waste heat recycling in vehicles and ‘off-grid’ homes, to heated clothing and other cold-climate amenities. We have been studying the arylazopyrazoles and azobispyrazoles in this regard, with several exciting outcomes. These include: (1) the discovery of a material that can store up to 92 kJ/mol of thermal energy for extended timescales (>2 weeks) while allowing for energy release at subzero temperatures, (2) the discovery of a material where energy release can occur electrocatalytically in the condensed phase, and (3) the discovery of materials with unprecedentedly large effective light penetration depths (1400 μm of UV at 365 nm and 1400 μm of visible light at 530 nm). Representative publications: J. Am. Chem. Soc. 2022, 144, 19430. DOI; J. Am. Chem. Soc. 2021, 143, 15250. DOI; J. Am. Chem. Soc. 2020, 142, 8688. DOI.
Circularly polarised light (CPL) is a form of chiral electromagnetic radiation, which theoretically should be able to induce absolute asymmetric synthesis via photochemical reactions. Indeed, the interaction of CPL with primordial interstellar molecules (combined with some amplification mechanism) is one hypothesis for the evolution of homochirality on Earth. As early as the 19th century van’t Hoff and Le Bel pointed out that CPL may be exploited to induce asymmetric photochemistry, however despite extensive efforts in the subsequent century, only a few examples exist with moderate levels of asymmetric induction, and no examples exist for highly enantioselective reactions. Nonetheless, CPL-mediated photochemistry continues to draw significant interest in asymmetric synthesis, origins of life science, asymmetric photoswitching of chiral molecules, molecular motors, and liquid crystalline materials.
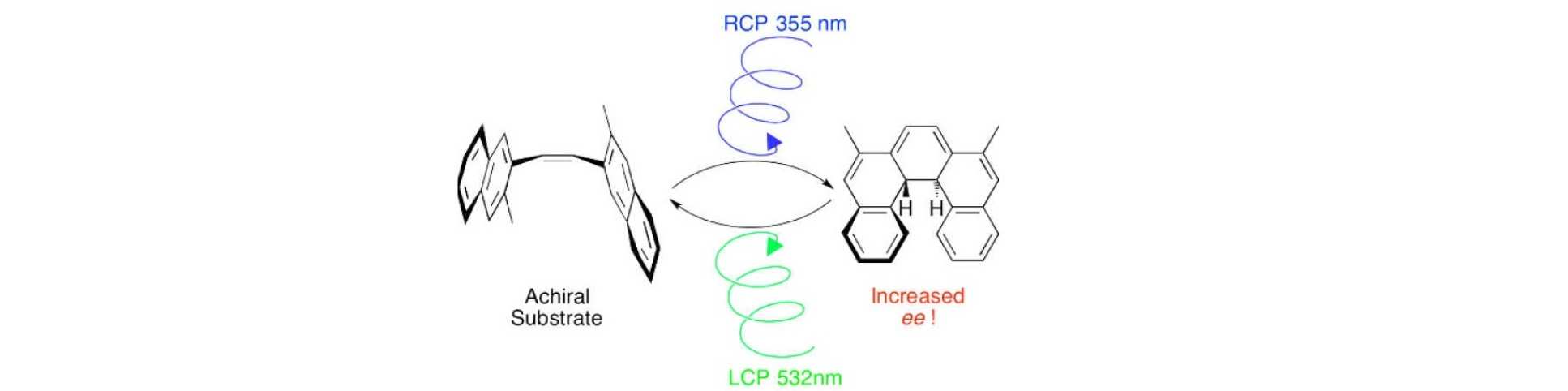
Building on our work studying the interaction of CPL with homochiral helicenes in photo-transistors, we have revisited the asymmetric photochemical synthesis of helicenes from a prochiral precursor using CPL in collaboration with Dr Marina Kuimova. We have demonstrated that two CPL wavelengths can control separate reactions simultaneously. In doing so, a photostationary state can be established such that the enantiomeric induction intrinsic to each step can combine additively, significantly increasing the asymmetric induction possible in these reactions. We continue to explore other mechanisms to enhance CPL photochemistry. Representative publication: Chem. Sci. 2015, 6, 3853. DOI.
Contact
Matthew J. Fuchter FRSC
Professor of Chemistry
Department of Chemistry
Molecular Sciences Research Hub, White City Campus
Wood Lane, London, W12 OBZ
m.fuchter@imperial.ac.uk
Tel: +44 (0)20 8594 5815