Ovarian cancer: fighting a silent killer
Words: Sarah Woodward
Context
Ovarian cancer is the sixth most common cancer in females, usually affecting women after the menopause or those with a family history of the disease. There are 6,000 new cases a year in the UK alone, but the long-term survival rate is just 35-40 per cent. Often called “the silent killer” due to the fact that many patients are diagnosed too late for effective treatment, there is an urgent need to find new ways to treat the disease.
Background
“With masses of data in our healthcare system, medical researchers are beginning to ask how such data can benefit patients through artificial intelligence (AI), while protecting patient privacy,” says Eric Aboagye, Professor of Cancer Pharmacology and Molecular Imaging. “It is against this background that a team of scientists at Imperial created an algorithm for predicting the prognosis of ovarian cancer patients.”
Methodology
In a study believed to be the largest in the world, Imperial researchers used a mathematical software tool known as TEXLab to discover patterns in routine diagnostic scans. They analysed CT scans and tissue samples taken between 2004 and 2015 from 364 women with ovarian cancer, to identify the aggressiveness of tumours. Using machine-learning techniques, the software examined more than 660 features and chose four that describe biological characteristics of the tumours – structure, shape, size and genetic makeup – to assess the patients’ prognoses. Patients were then given a score known as Radiomic Prognostic Vector (RPV), ranging from mild to severe, to indicate how advanced the disease is.
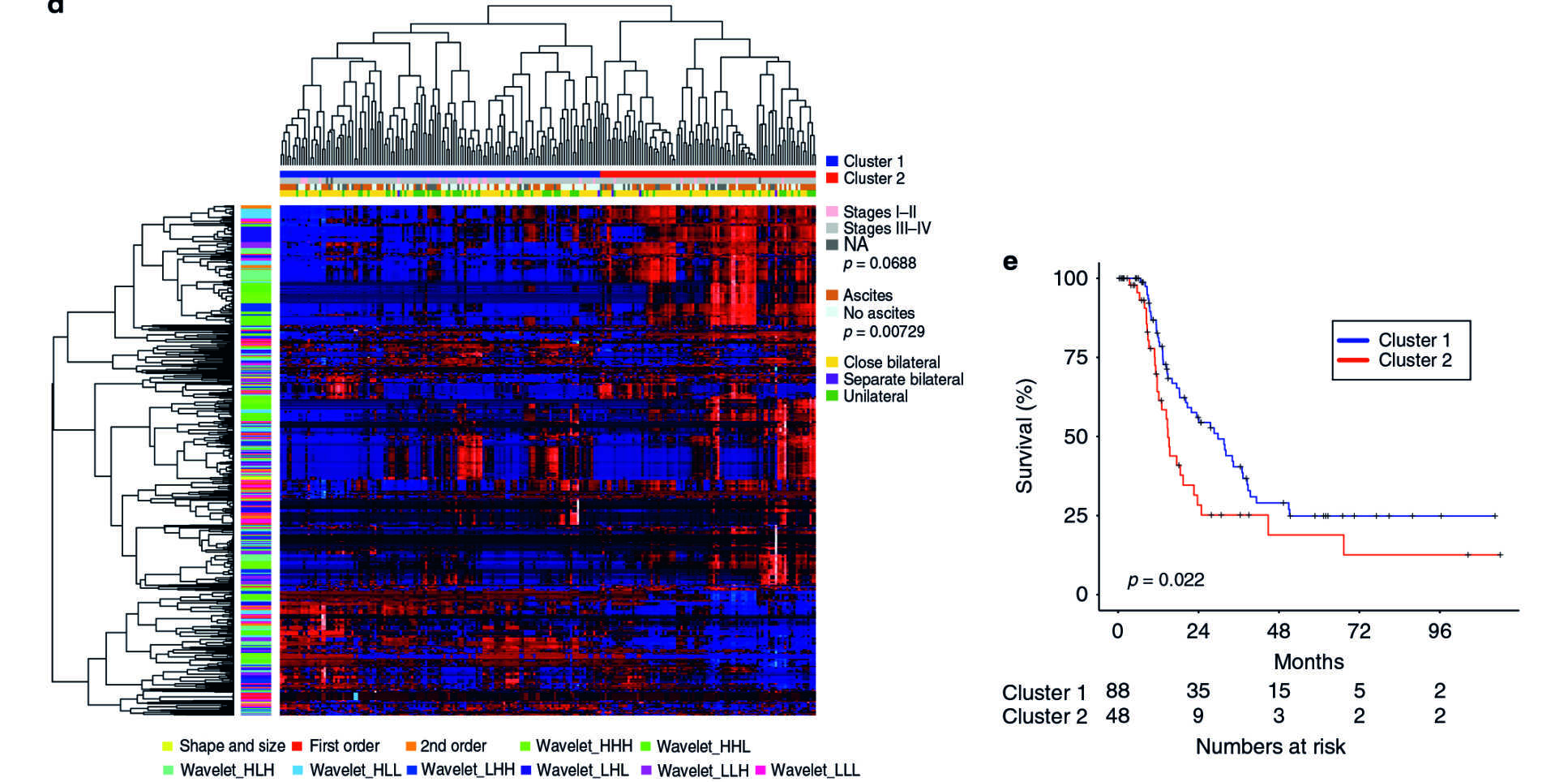
Findings
The software was up to four times more accurate in predicting deaths from ovarian cancer than the standard methods of blood tests – which look for a substance called CA125, an indication of cancer – and CT scans. The team found that five per cent of patients with high RPV scores had a survival rate of less than two years. High RPV was also associated with chemotherapy resistance and poor surgical outcomes, suggesting that RPV can be used as a potential biomarker to predict how patients will respond to treatments.
Outcomes
In conjunction with the pharmaceutical industry, the team is working on how AI can be used to predict which patients will benefit from specific drugs, including immunotherapy. “Although each patient’s experience is unique, surgery and chemotherapy for late-stage ovarian cancer can have significant side-effects,” says Aboagye. “However, we can now identify those patients unlikely to benefit from such standard treatments and offer them alternatives. Our technology is able to give clinicians more accurate information on how patients are likely to respond to treatments, which could enable them to make better and more targeted treatment decisions.”
The next step is to further appraise the RPV test to make it usable in the NHS environment, necessitating a larger study to see how accurately the software can predict the outcomes of surgery and/or drug therapies for individual patients. But Aboagye has wider ambitions. He says: “In the future we hope to be able to apply similar methodology to other diseases, including lung and brain cancers, as well as Alzheimer’s disease.”
Professor Eric Aboagye is Professor of Cancer Pharmacology and Molecular Imaging in the Department of Surgery and Cancer.