Development of antagonists of the human IgE-FceRI protein-protein interaction

Human allergic disorders (e.g. asthma, hay fever, atopic dermatitis) are increasing world-wide and presently there are no effective treatments. Clinical manifestations are the end product of a complex allergic cascade initiated by cross linking of receptor bound immunoglobulin E (IgE) on the surface of mast cells by cognate antigens. Inhibition of the initial binding between the IgE ligands and the high-affinity (FceRI) receptors on the mast cells is a possible therapeutic strategy.
Various disulfide looped peptides containing the receptor binding epitopes are antagonists of the interaction and elicit the production of antibodies which inhibit the binding of IgE to the receptor but do not activate IgE sensitised cells. As peptides generally lack the oral and cellular bioavailability required for therapeutic activity we are presently developing a series of non-peptide 'scaffold' molecules to replace the crude conformational constraint afforded by the disulfide bridge present in some of these peptide loops. Covalent attachment of the peptide binding motif to the scaffold should result in it being constrained into conformations which resemble that in IgE. Systematic site-specific 'synthetic mutagenesis' of these compounds should allow us to iteratively optimise the most promising compounds by imposing further conformational restraints on the peptide by way of residue replacements.
This work is being carried out in collaboration with Robin Leatherbarrow (Chemistry, IC) and Brian Sutton and Andrew Beavil (Structural Biology, KCL).
Funded by
Collaborations with
- Dr Birgit Helm for molecular biology of IgE (University of Sheffield, Department of Molecular Biology and Biotechnology, Sheffield, UK). Prof Robin Leatherbarrow for peptide and protein chemistry (Imperial College London, Department of Chemistry, South Kensington Campus, London, UK). Prof Brian Sutton for structural biology and protein crystallography of IgE and its receptor (Kings College London, Randall Institute, Guy's Hospital Campus, London, UK). Dr Andrew Beavil for structural and molecular biology of IgE and its receptor (Kings College London, Randall Institute, Guy's Hospital Campus, London, UK).
Selected Publications
- D.A. Offermann, J.E. McKendrick, J.J.P. Sejberg, B. Mo, M.D. Holdom, B.A. Helm, R.J. Leatherbarrow, A.J. Beavil, B.J. Sutton, A.C. Spivey, 'Synthesis and Incorporation into Cyclic Peptides of Tolan Amino Acids and their Hydrogenated Congeners: Construction of an Array of A-B-loop Mimetics of the Cε3 Domain of Human IgE', J. Org. Chem. 2012, 77, 3197-3214 [(2.39MB)];
- I. Sayers, J.E.M. Housden, A.C. Spivey, B.A. Helm, ' The importance of Lys-352 of human immunoglobulin E in FceRII/CD23 recognition' J. Biol. Chem. 2004, 279, 35320-35325. [PDF(272KB)];
- A.C. Spivey, J. McKendrick, R. Srikaran, B.A. Helm 'Solid-phase synthesis of an A-B loop mimetic of the Ce3 domain of human IgE: Macrocyclization by Sonogashira coupling' J. Org. Chem. 2003, 68, 1843-1851. [PDF(188KB)];
- B.A. Helm, I. Sayers, J. Swan, L.J.C. Smyth, S.A. Cain, M. Suter, D.C. Machado, A.C. Spivey, E.A. Padlan, 'Protein and cell engineering of components of the human immunoglobulin E receptor/effector system: applicatios for therapy and diagnosis', Technology and Health Care, 1998, 6, 195-207. [PDF (2,583KB)]; See also: Publications
Development of small molecule regulators of the nuclear receptor LRH-1
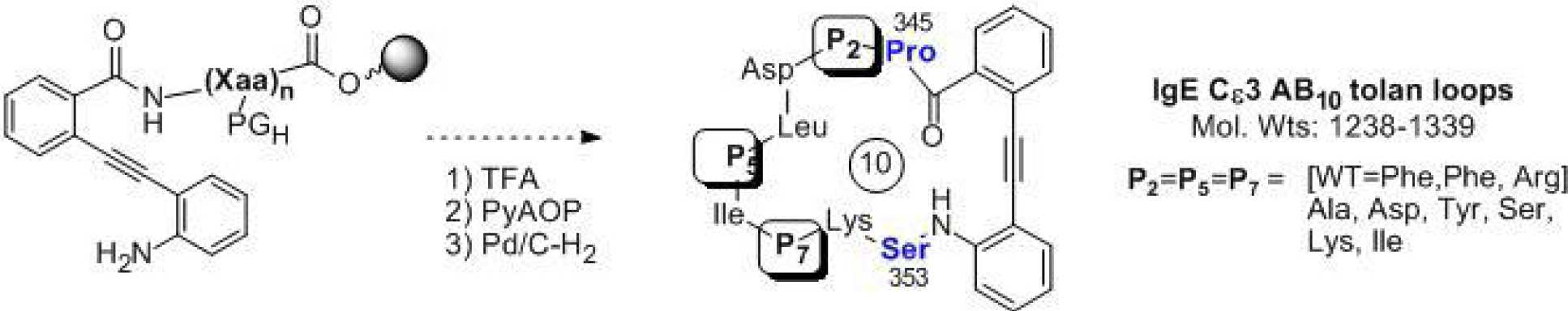
Nuclear receptors (NR’s) represent a major target for the treatment of human disease. Development of modulators for many NR’s has resulted in powerful treatments for many therapeutically important conditions. Traditional therapies of NR’s target the ligand binding pocket of the ligand binding domain (LBD) although resistance to these therapeutics has been observed. We are using in silico and screening techniques to develop antagonists/inverse agonists of the interaction of various coactivator proteins with the nuclear receptor LRH-1 as an approach to the discovery of new cancer therapeutics.
Funded by
Collaborations with
- Prof Simak Ali for nuclear receptor biology (Imperial College London, Department of Oncology, Hammersmith Campus, London, UK).
Selected Publications
- P.T Thiruchelvam, C.F. Lai, H. Hua, R.S. Thomas, A. Hurtado, W. Hudson, A.R. Bayly, F.J. Kyle, M. Periyasamy, A.Photiou, A.C. Spivey, E.A. Ortlund, R.J Whitby, J.S. Carroll, R.C. Coombes, L. Buluwela, S. Ali 'The liver receptorhomolog-1 regulates estrogen receptor expression in breast cancer cells' Breast Cancer Res. Treat. 2010, 127, 38 5-396 [PDF (775KB)];
- A.C. Spivey, L. Laraia, A.R. Bayly, H.S. Rzepa, A.J.P.White ' Synthesis of cis- and trans-2,3-DisubstitutedTetrahydrofurans via Oxonium-Prins Cyclization – Access to the Cordigol Ring System' Org. Lett. 2010, 12, 900-903 [PDF (420KB)]; See also: Publications
Investigation of the interaction of nitric oxide with protein thiols
Nitric oxide (NO) mediates a vast array of cellular functions. One mechanism by which this occurs isvia direct reaction of NO (or derivatives thereof) with protein thiols. We have an interest in delineating the structural features which predicate peptide cysteine thiols to this post-translational modification. We have previously explored this using several 'one bead one peptide' libraries using combinatorial 'split and mix synthesis'. Following exposure of the libraries to a limited dose of NO, NOx, or various NO-carriers the beads are assayed by chemical derivitisation which allows physical separation of hits, comprising eitherS-nitrosothiols or sulfenic acid derivatives. deconvolution should reveal protein primary sequence motifs which constitute sites for specific post-translational modification of cysteine residues for signal transduction.
We are currently exploring how specific oxidative modifications of Human Serum Albumin (HSA) correlate with clinical outcomes for critically ill patients with severe respiratory and hepatic disorders.
Funded by
Collaborations with
- Dr Greg Qunilan for oxidative stress in respiratory critical care (Imperial College London, The Brompton Hospital, London, UK). Dr Nathan Davies for oxidative stress in hepatic disorders (University College London, Institute of Hepatology, London, UK).
Selected Publications
- A.C. Spivey, J. Colley, L. Sprigens, S.M. Hancock, D.S. Cameron, K.I. Chigboh, G. Veitch, C.S. Frampton, H. Adams, 'The synthesis of water soluble decalin-based thiols and S-nitrosothiols model systems for studying the reactions of nitric oxide with protein thiols' Org. Biomol. Chem. 2005, 3, 1942-1952. [PDF (348KB)];
- E.A.G. Demoncheaux, T.W. Higenbottam, D.G. Kiely, J-M. Wong, S. Wharton, R. Varcoe, T. Siddons, A.C. Spivey, K. Hall, A.P. Gize 'Decreased whole body endogenous nitric oxide production in patients with primary pulmonary hypertension' J. Vasc. Res. 2005, 42, 133-136. [PDF 109KB)];
- E. Demoncheaux, D. Crowther, A.C. Spivey, T.W. Higenbottam, 'Monitoring reactive nitrogen species in biological milieu: a difficult journey', Am. J. Resp. Crit. Care Med. 2002, 165, 1670-1672. [PDF (98KB)]. See also: Publications
Development of 18F labelled EGFR receptor imaging agents for PET
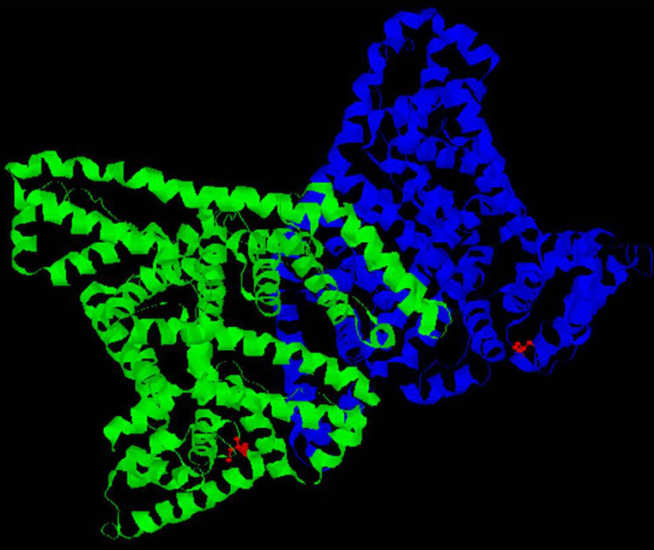
The Epidermal growth factor receptor (EGFR/c-ErbB1/HER1) is overexpressed in breast, ovarian and other human cancers. EGFR is a transmembrane glycoprotein with an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain with tyrosine kinase activity. EGFR is activated by binding to a variety of ligands including EGF, amphiregulin and TGF-alpha. Once activated it is believed to undergo homo- or heterodimerisation followed by activation of the intrinsic protein tyrosine kinase, autophosphorylation and activation of intracellular signal transduction pathways such as phosphatidylinositol-3-kinase (PI3K)/AKT and ras/raf/MEK/MAPK.
The blockage of EGFR activity therefore appears to be a potent strategy in the treatment of cancer, and various anti-EGFR therapies have been developed. Some small molecule inhibitors have already been approved for clinical use. Unfortunately, clinical use of these inhibitors has led to the emergence of drugs resistant tumours via gene amplification or mutation in the kinase domain. Molecular imaging techniques, such as Positron Emission Tomography (PET) have the potential to provide insights into EGFR biology. PET can non-invasively determine whether the target protein is overexpressed in a specific primary tumour or metastasis in vivo, the magnitude and duration of receptor occupancy, and target-drug interactions (including potentially the functional consequence of mutations that lead to reduced EGFR-drug interactions).
We are currently working with Eric Aboayge (IC, Oncology, Hammersmith) developing methods for 18F labeling of certain heterocyclic compounds for imaging of breast and other cancers.
Funded by
Collaborations with
- Prof Eric Aboagye for PET imaging (Imperial College London, Department of Oncology, Hammersmith Campus, London, UK).
Selected Publications
- F. Pisaneschi, Q.-D. Nguyen, E. Shamsaei, M. Glaser, E. Robins, M. Kaliszczak, G. Smith, A.C. Spivey, E.O. Aboagye 'Development of a new epidermal growth factor receptor positron emission tomography imaging agent based on the3-cyanoquinoline core: Synthesis and biological evaluation' Bioorganic & Medicinal Chemistry 2010, 18, 6634-6645 [PDF (873KB)];
- H.L. Evans, R.L. Slade, L. Carroll, G. Smith, Q.-D. Nguyen, L. Iddon, N. Kamaly, H. Stöckmann, F.J. Lee per, E.O. Aboagye, A.C. Spivey, 'Copper-free Click – A Promising Tool for Pre-Targeted PET Imaging', Chem. Commun. 2012, 991-993 [PDF (1140KB)]; see Publications
Contact details
Email: a.c.spivey@imperial.ac.uk
Skype: alan.spivey
Tel & Fax: +44 (0)20 75945841
Further links: