We use adapted for Design and Development of medical devices within our Team which we use to support the projects, with a level of support adapted to the project’s TRL and RRL (regulatory readiness level), see Figure 3. The latter is a tool that we have developed and are trialling.
TRL- for Design and Development of medical devices within our Team which we use to support the projects, with a level of support adapted to the project’s TRL.
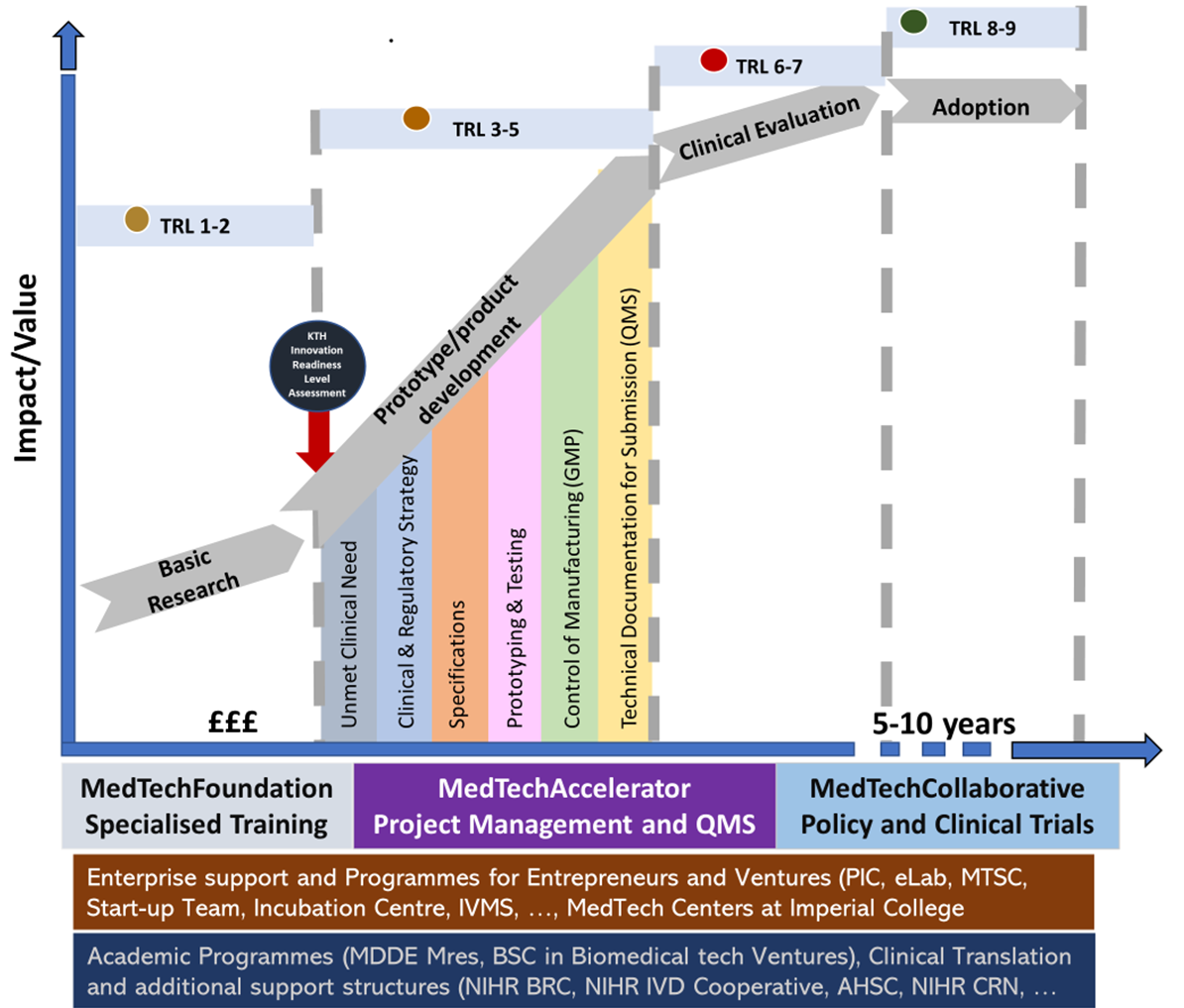
Through our Foundation and Collaborative stream links, colleagues at the NIHR IVD Cooperative and the newly formed NIHR BRC Bioengineering Theme, we are also able to support clinical translation focused projects. Importantly, we also encourage the entrepreneurs to participate in Entrepreneurship development programmes, in particular the Imperial led MedTech Superconnector programme.
The accelerator programme is based on two previous successful pilot models, ran at Centre level, which have generated 4 Imperial College Start-ups, 4 RAEng Enterprise Fellowships, and led to more than £3.3m in translational funding being raised for projects over 4 years. We have expanded the model, to encompass a wider gamut of clinical application areas across College.
